Validation of Gate2Brain Technology in the Transport of Antibodies to the Brain Opens the Avenue of the Next Generation of CNS Therapeutics with Improved Delivery to the Brain
Image: Marina Plaza
Press release
New research highlights how advanced peptide shuttles—the core of Gate2Brain technology platform—are poised to revolutionize drug delivery across the blood–brain barrier of therapies such as the ones based on antibodies that although being promising many times are limited due to poor accessibility to the brain.
Two state-of-the-art articles, published in Molecular Pharmaceutics, have put the focus and robustly validated the versatility of Gate2Brain technology platform—a system capable of delivering a wide range of therapeutic agents across the blood–brain barrier (BBB). These findings demonstrate that, whether delivering monoclonal antibodies or other drug modalities, the innovative brain shuttle peptides at the core of the Gate2Brain platform can overcome one of modern medicine’s greatest challenges.
Key Highlights of the Research:
- Enhanced BBB Transport and Targeted Therapy:
The study titled“A Site-Specific MiniAp4–Trastuzumab Conjugate Prevents Brain Metastasis”—authored by Mariam Masmudi-Martín, María Perea, Manuel Valiente, Ernest Giralt, Macarena Sánchez Navarro and Benjamí Oller-Salvia, including Meritxell Teixidó CEO and CSO of Gate2Brain—demonstrates that conjugating MiniAp-4, a protease-resistant BBB shuttle peptide, to trastuzumab significantly enhances its transport across the BBB. This novel antibody–shuttle conjugate markedly reduces both the number and size of metastases, including clinically relevant large metastases. This finding opens the way to the therapeutic use of antibodies and antibody-drug conjugates for CNS diseases without the bottleneck that the blood-brain barrier represents. - Versatile Drug Delivery Platform:
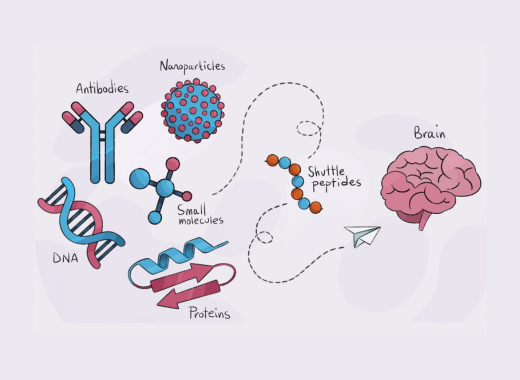
The review article“New Trends in Brain Shuttle Peptides”, by Roger Prades, Meritxell Teixidó, and Benjamí Oller-Salvia, highlights the adaptability of peptide shuttles in transporting a diverse range of therapeutics—from small molecules to proteins and nanoparticles. This work details how recent advances in peptide engineering contribute to a more efficient, receptor-mediated transcytosis process, thereby validating the superior performance of the Gate2Brain platform. - Potential for Broader Applications:
Both studies underscore the transformative potential of peptide shuttles in revolutionizing brain-targeted drug delivery. The findings open new avenues for treating central nervous system diseases—including brain metastases, glioblastoma, Alzheimer’s, and other neurodegenerative disorders—further validating the Gate2Brain approach. We are in front of the next generation of tools to cross the BBB and efficiently deliver drugs to the brain.
Advancing Drug Delivery Across the BBB with Gate2Brain Technology
The first study provides compelling evidence that precise chemical modifications enable the targeted delivery of large biomolecules to the brain. The MiniAp-4–trastuzumab conjugate not only maintains therapeutic efficacy but also achieves significantly improved brain penetration, demonstrating a protective effect against brain metastasis in preclinical models. These results validate the Gate2Brain technology platform, suggesting that MiniAp-4 could potentially protect the brain from metastases by improving the delivery of drugs to protect it.The second study offers a comprehensive review of the evolution and innovation in brain shuttle peptides over the past decade. It emphasizes the advantages of receptor-mediated transcytosis (RMT) and the latest strategies in peptide engineering to enhance drug delivery across the BBB. This work further substantiates the Gate2Brain platform’s ability to efficiently transport a broad spectrum of therapeutic agents.
Together, these studies reinforce the concept that advanced peptide-based brain shuttle technologies—central to the Gate2Brain platform—are dynamic and adaptable tools for overcoming the challenges of brain drug delivery. This breakthrough holds great promise for accelerating the clinical translation of new treatments for a wide range of neurological disorders, where there is a candidate drug that needs a better transport. Giving an opportunity to all those suffering from brain diseases.
Publications:
- A Site-Specific MiniAp4–Trastuzumab Conjugate Prevents Brain Metastasis”
- “New Trends in Brain Shuttle Peptides”
About Gate2Brain
Gate2Brain is a technology platform spin off from IRB Barcelona, the University of Barcelona, and the Sant Joan de Déu Hospital, founded in 2020. Gate2Brain uses its proprietary peptide-based technology to cross biological barriers, such as the blood–brain barrier, to improve drug delivery to the brain and reduce side effects. With a first asset in pediatric brain tumors the present work demonstrates the expansion of the potential use of their technology.
The company has received support from institutions including Fundación Botín (Mind the Gap), Banco Sabadell (BStartup Health), CDTI- Ministerio de Ciencia e Innovación, European Union (Next Generation EU), the European Innovation Council (EIC Accelerator), Startup Capital d’ACCIÓ, and CaixaResearch Consolidate de la Fundació “la Caixa”.
Gate2Brain envisions improving patients’ quality of life through technology that enhances drug delivery efficiency.
With the Support of:

Contact Information:
Meritxell Teixidó
Gate2Brain
info@gate2brain.com
www.gate2brain.com

 G2B
G2B


 gate2brain
gate2brain





