Gate2Brain, beyond barriers. An interview by Oncobites
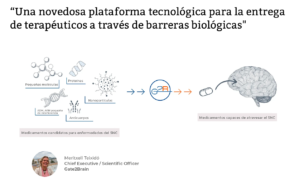
A novel technological platform for the delivery of therapeutics across biological barriers
Meritxell Teixidó is a chemist, PhD in Peptide Science and Genetic Algorithms, Executive MBA in Entrepreneurship and Innovation. Throughout his career, he has contributed to the advancement of science in drug delivery technologies and transport studies across the blood-brain barrier by publishing more than 50 articles and obtaining 6 patents. In addition, it has played a crucial role in training new talent. She is the CEO and Chief Scientist of Gate2Brain.
Question: How did the idea to create Gate2Brain come about?
Meritxell Teixidó: After directing for 15 years at the Institute for Research Bioscience (IRB) Barcelona the line of research that has been the seed of the Gate2Brain technology, I saw that the results of the 10 doctoral theses that I co-directed allowed me to create a solid technology , backed by robust intellectual property on which to build a biotech company to develop the first indication that would benefit from the technology.
This indication is in pediatric oncology, specifically in pediatric brain tumors with an intact blood-brain barrier, where we could contribute to prolonging patient survival by improving the transport of drugs to the brain. We enrolled in the Mind the Gap (MtG) program of the Botín Foundation and BStartup Health of Banco Sabadell. These were the first economic boost in this entrepreneurial path for which I prepared for years, assuming the role of CEO/CSO, and in 2020 I left academia.
Personally, I wanted to experience first-hand how far we could go with our results, dreaming that we would change the prognosis of a brain disease by improving the transport of a drug candidate that does not arrive effectively alone.
Starting with pediatric oncology was a decision based on multiple factors (unmet medical need, betting on the design of drugs for the pediatric population, access to animal models derived from patients, having Hospital Sant Joan de Déu – Barcelona as Co-founder) , then the technology would be more validated and could make the leap to other indications. Keeping in mind that 1 in 4 of us need brain treatment during our lives and although there are drug candidates for almost all of these diseases, 98% of these do not cross the barrier without technology to help them and this stops their development and the opportunity for patients.
Q: What inspired you to create the technology to cross through biological barriers?
MT: In nature we find peptides (small proteins) that are capable of crossing the blood-brain barrier that protects the brain. We were inspired by these and their potential use as a tractor to carry drugs (like trailers) that cannot reach them effectively on their own. We modified these tractor peptides so that they were stable to bloodstream proteases and we benefited from the fact that their transport mechanism does not affect the normal functioning of the barrier or its protective role.
Q: What is Gate2Brain technology?
MT: Gate2Brain is a patented technology based on peptides that allows drugs of a different nature (small molecules, nanoparticles, antibodies, proteins) to be delivered to the brain parenchyma that are not capable of achieving it without help.
These drugs are covalently linked to our tractor peptides and this new chemical entity that is created, the peptide-drug conjugate for which all preclinical and clinical development must be carried out, also represents an opportunity to create new intellectual property.
Once in the brain, the technology is also prepared for cases where the drug must be separated from the tractor peptide.
Our flagship product, G2B-002, uses Gate2Brain technology to deliver a chemotherapeutic agent to the brain for a therapeutic tool for intact barrier pediatric brain tumors such as diffuse pons glioma (DIPG) and pediatric glioblastoma (pGBM). .
G2B-002 is in the stage of preclinical studies in patient-derived animal models. With a dream, reaching a clinical trial in 2026, this trial would open the door to moving towards the market and giving an opportunity to patients with these incurable tumors and their families.
Q: What are the technological features that Gate2Brain offers for cancer therapeutics?
MT: G2B-002 is chemically prepared and is a scalable solution, so we could imagine that in the future it could benefit other patients with brain tumors sensitive to the transported drug, both in the pediatric and adult populations.
The tractor peptide, which has the role of directing and allowing transport to the brain, reduces side effects compared to more targeted therapies.
Q: What benefits can clinicians expect by recognizing Gate2Brain technology in their daily practice?
MT: Our mission is to have better treatments for brain tumors, optimizing their transport to the brain.
Q: What clinical evidence supports technology across biological barriers for cancer treatment? What have you discovered in the preclinical phase, what do you hope to find?
MT: Our product G2B-002 is in regulatory preclinical 2-3 years from a PhI/IIa clinical trial. In preclinical studies, it is worth highlighting its good tolerability in vivo and its effectiveness in transporting to the brain in rodents.
Q: What are the challenges generated when executing your technology studies and how have you addressed them?
MT: Choosing which would be the first indication that would benefit from the technology led me to a multifactorial analysis carried out by a multidisciplinary team that allowed me to approach pediatric oncology. This choice was based on several reasons: unmet medical need, a completely intact barrier in these childhood brain tumors, possibility of requesting orphan drug designation, being able to carry out the phase I/IIa clinical trial at the Sant Joan de Déu Hospital. . But it also involved challenges and being brave in the search for investment, obstacles that we have been overcoming, and a paradigm shift has also been generated in which we are increasingly betting on drugs with a development path from pediatric to adult. and investment funds focused on pediatrics begin to operate, such as the Montana Impact Fund. Recently, we have participated and presented our project at the European Pediatric Innovation Day in Warsaw and we are part of networks such as i4kids.
Q: What message or advice do you want to share with the oncology community about the importance of the technology you provide?
MT: Technologies like Gate2Brain can improve the transport of drugs to the brain and thus help to have better therapies for brain tumors in the coming years, starting first with childhood DIPG and pGBM patients.





